45 mock up labels and packages certification form
How to Correctly Fill the Online PMP Application Form? Once you have registered an account on the PMI website, you will be able to find the online application form for the PMP® Certification under "myPMI" section.. Filling the online PMP® application form is a project in itself. In addition to filling the personal particulars and details, PMI requires you to record your Project Management Experiences in details which requires you a lot of ... Medicines: packaging, labelling and patient information ... Submission of notifications for self-certified changes to labels and/or leaflets must use either the notification application form (MS Word Document, 324KB) in Word format or the portal form in PDF...
Custom Packaging Design | Package Printing | Vaughan ... Once the product launch has been set, we deliver the products to your suppliers if required. We offer various affordable delivery options that can accommodate your product launch in Brampton, Mississauga, and Vaughan. Communication. At Printing Box, we follow a deliverable schedule that outlines the various stages of the product launch listing ...

Mock up labels and packages certification form
Mock-Up-Guidance Document - BASG To summarize legal provisions, requirements regarding content as well as information how to submit and handle Mock-Ups a new Guidance Document was set up, which can be found under (currently only available in German). Further inquiry note basg_anfragen@basg.gv.at PDF The Challenge Design Criteria - paperboardpackaging.org The package must be commercially producible on standard machinery. Any hand assembly should be . minimal and purposeful. Including decorations like . magnets, ribbons, etc. that need to be attached to the package make it difficult to mass produce. The package should be designed as ready-to-ship with a label or include an outer shipping package. PDF Certification of Origin Form (USMCA/CUSMA/T-MEC) - UPS Certification of Origin Form (USMCA/CUSMA/T-MEC) ... Include the period if the certification covers multiple shipments of identical goods for a specified period of up to 12 months. 3. Certifier Provide the certifier's name, title, address (including country), telephone number, and email address. 4. Exporter
Mock up labels and packages certification form. Changing the labelling and package leaflet (Article 61(3 ... Certifying medicinal products Changing the (invented) name of a medicinal product Changing the labelling and package leaflet (Article 61 (3) notifications) Guidance Classifying post-authorisation changes Compliance Contacting EMA: post-authorisation Data on medicines (ISO IDMP standards) Improving quality of submissions Packaging Design for Beginners: How to Create a Simple Box Go to File > Place, and choose a pattern from the Color splash patterns pack you downloaded earlier. Click Open, and allow the image to fill up the whole frame. Step 2 Use the Eyedropper Tool (I) to pick up the very pale pink color from the pattern. Double-click on the Fill Color box at the bottom of the Tools panel to open the Color Picker window. PDF Appendix 9: Labelling Requirements 9.1.1 LABEL (MOCK-UP) FOR IMMEDIATE CONTAINER AND OUTER CARTON Please refer to Figure 1as an example of a product label which in accordance to the labelling requirements. Drug Registration Guidance Document (DRGD) National Pharmaceutical Regulatory Division, Ministry of Health Malaysia. Second Edition, Sept 2016. Revised July 2018 Page | 416 PDF (~ II U.S. - accessdata.fda.gov requesting advisory comments, the proposed materials in draft or mock-up form with annotated references, and the package insert (PI), Medication Guide, and patient PI (as applicable) to: OPDP Regulatory Project Manager Food and Drug Administration Center for Drug Evaluation and Research Office of Prescription Drug Promotion 5901-B Ammendale Road
DOCX Canada Mock-Up Label s and Packages Certification Form for Non-p rescription Drugs and Contact Lens Disinfectants Protected A DRUG PRODUCT INFORMATION Submission Type Brand, Proprietary or Product Name (as per Field #8 on the Drug Submission Application Form) PDF US EPA, Pesticide Product Label, VANITY GP, 06/03/2014 • EPA Form 8570-1 • Red-lined copy of the master label, two copies • Clean copy of the master label • Mock-up of graphics label c- c c c (-c i: r l t <. c c L . If there is any other information that the agency needs to complete this submission, please feel free to contacc c < c t mVdirectly at PDF Instructions Form Requirements - GRANTS.GOV 04/20183.1 Form Mock-upForm Requirements 3.2 Burden Statement 3. Form Mock-up and Burden Statement4. * Form Instructions (Please use the following template to create detailed form instructions, then attach using Add Get Permanente Advantage Authorization ... - US Legal Forms Open the template in our feature-rich online editing tool by clicking on Get form. Fill out the requested boxes which are colored in yellow. Click the green arrow with the inscription Next to jump from box to box. Use the e-signature tool to e-sign the form. Add the date.
Packaging and Labeling - Food and Drug Administration a) Cut labeling - single labels for individual drug products that are ³cut´ from a sheet or roll of labels b) Cut labeling operations shall include one of the following: Design Your Own 'Custom Packaging' - Printing Circle Custom Packaging. Custom packaging is free of any limitation related to the shapes or sizes of the product. Whether it's big, small, or odd-shaped, cardboard, kraft paper, and corrugated cardboard are used to give the shapes to the boxes that can perfectly fit these products inside. The use makes these boxes a die-cutting technique that cuts ... Dodd Frank Certification Form - SignNow Enter your official contact and identification details. Use a check mark to point the answer wherever demanded. Double check all the fillable fields to ensure total accuracy. Utilize the Sign Tool to create and add your electronic signature to signNow the Dodd frank certification form. Press Done after you finish the blank. PDF Labels and Packages Certification Form for Prescription ... Labels and Packages Certification Form for Prescription Products Author: Government of Canada - Health Canada Subject: Labels and Packages Certification Form for Prescription Products Created Date: 12/2/2020 10:52:32 AM

Canada Labels and Packages Certification Form for Prescription Products Download Fillable PDF ...
Medicines: apply for a variation to your ... - GOV.UK Product information includes the summary of product characteristics, leaflets and labels. With a CCC you can: submit only one consolidated mock-up of the leaflets and labels with all the changes...
How to Print Labels From Excel - EDUCBA Step #4 - Connect Worksheet to the Labels. Now, let us connect the worksheet, which actually is containing the labels data, to these labels and then print it up. Go to Mailing tab > Select Recipients (appears under Start Mail Merge group)> Use an Existing List. A new Select Data Source window will pop up.
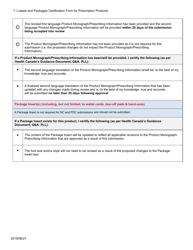
Canada Labels and Packages Certification Form for Prescription Products Download Fillable PDF ...
PDF NDS/SNDS Screening Report (Including response to ... - CAPRA Are labels required for this submission, according to the Mock-Up Labels and Packages Certification form (actual size for all strengths, dosage forms & proposed packaging formats)? If yes, have mock-ups been provided: In English: ☐ Clean ☐ Annotated
PDF ANDA 206604 ANDA APPROVAL - Food and Drug Administration requesting advisory comments, the proposed materials in draft or mock-up form with annotated references, and the package insert (PI), Medication Guide, and patient PI (as applicable) to: OPDP Regulatory Project Manager Food and Drug Administration Center for Drug Evaluation and Research Office of Prescription Drug Promotion 5901-B Ammendale Road
PDF Labels and Packages Certification Form for Prescription ... A mock-up of only the smallest label and/or package for each dosage form and strength has been provided in both official languages, as • there are no differences other than pill count or volume on the labels/packages; and • all the other labels/packages will have identical text, format, size, layout, color, etc.
PDF Labels and Packages Certification Form for Non ... - Canada A mock-up of only the smallest label and/or package for each dosage form and strength has been provided in both official languages, as • there are no differences other than pill count or volume on the labels/packages; and • all the other labels/packages will have identical text, format, size, layout, color, etc.
Forms: Applications and submissions for drug products - Canada Labels and Packages Certification Form for Prescription Products [2020-12-21] Master File (MF) Application Fee Form for Human Drugs [in effect April 1 2022] (DOC Version - 55 KB) Non-prescription drug monograph attestation form (PDF fillable/saveable - 648 KB) [2016-01-15]
In Year Casual Application Form - US Legal Forms Open it up with cloud-based editor and start adjusting. Complete the empty fields; concerned parties names, places of residence and phone numbers etc. Change the template with smart fillable fields. Include the particular date and place your electronic signature. Click Done following double-checking all the data.
25 Best Packaging Design Courses Online (Free & Paid) Product Packaging Design And Mockup: Creative Level — Best for Mockups All 25 packaging design courses listed below Free Graphic Design Software Trial + Adobe CC Discount If you're going to become a professional package designer, a subscription to the industry standard software, Adobe Creative Cloud is recommended.
PDF Certification of Origin Form (USMCA/CUSMA/T-MEC) - UPS Certification of Origin Form (USMCA/CUSMA/T-MEC) ... Include the period if the certification covers multiple shipments of identical goods for a specified period of up to 12 months. 3. Certifier Provide the certifier's name, title, address (including country), telephone number, and email address. 4. Exporter
PDF The Challenge Design Criteria - paperboardpackaging.org The package must be commercially producible on standard machinery. Any hand assembly should be . minimal and purposeful. Including decorations like . magnets, ribbons, etc. that need to be attached to the package make it difficult to mass produce. The package should be designed as ready-to-ship with a label or include an outer shipping package.






Post a Comment for "45 mock up labels and packages certification form"